Photodegradation
of Polymers
Polymers will change over time when exposed to UV radiation. These changes are the result of light-induced homolytic fission of chemical bonds (photolysis) and photo-oxidation.
The UV resistance of unprotected polymers varies widely and depends on the structure and composition. In general, polymers with high bond energies and without UV sensitive groups are very stable, whereas those with weak bonds and high concentration of chromophoric groups1 are very sensitive to UV degradation. Another important factor is the accessibility of reactive hydrogen on secondary and tertiary carbon atoms. In the case of polyolefins, the UV stability decreases in the order1
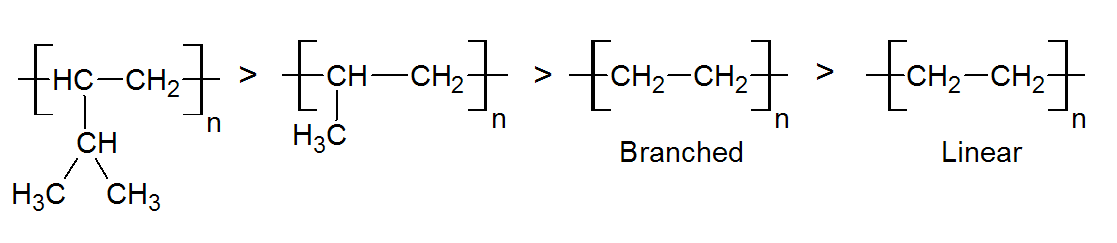
The UV stability will also depend on the concentration and type of
impurities like residual catalyst and thermal degradation by-products
from melt processing of the resin. These contaminations can either
directly initiate photo degradation or can sensitize the polymer. The addition of pigments and fillers will
usually improve the UV stability. For example, in the case of
carbon black, the degradation rates of black PE films vary directly
with particle size and inversely with concentration.2
Typical initiators for photo degradation are ketones, quinones and peroxides because they absorb light below 400 nm which causes bond excitation and cleavage to radicals. Thus, photo degradation may occur in the absence of oxygen but will be greatly accelerated by oxygen.
Photo degradation usually starts at the surface with visible cracks and discoloration, which leads to rapid loss of mechanical properties. In theory, olefins such as polyethylene (PE) and polypropylene (PP) are stable when exposed to sunlight, because both C-C and C-H bonds do not absorb UV radiation from the sun. However, there are often contaminations present which can initiate photo degradation resulting in surface cracking and loss of mechanical properties.
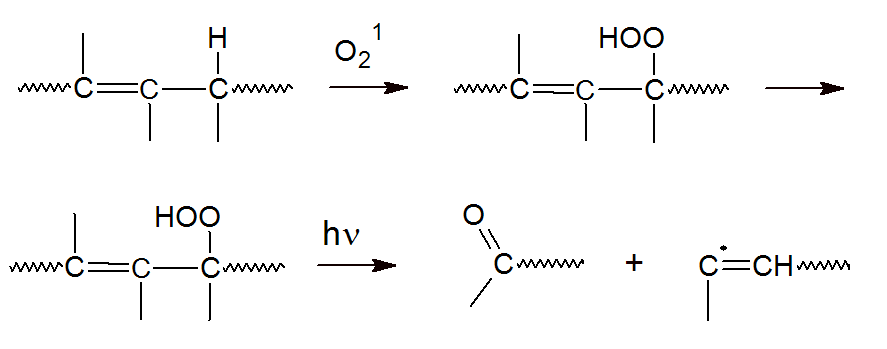
There are several mechanisms that can contribute to photo degradation. For example, if free radicals are directly produced by UV radiation, then all subsequent reactions are similar to those of thermal degradation, including chain scission, crosslinking, and secondary oxidation. Another mechanism of photo oxidation includes the photochemical production of electronically excited oxygen. In this case, degradation takes place by direct oxidation, for example, by reaction with allylic hydrogen which then could undergo chain scission with formation of terminal ketone groups:
Another contributor to photo degradation is ketone photolysis which proceeds via Norrish reaction. Ketones are formed by either thermolysis or photolysis of hydro peroxides. If these groups are exposed to light, they undergo chain scission via Norish I or II reaction:
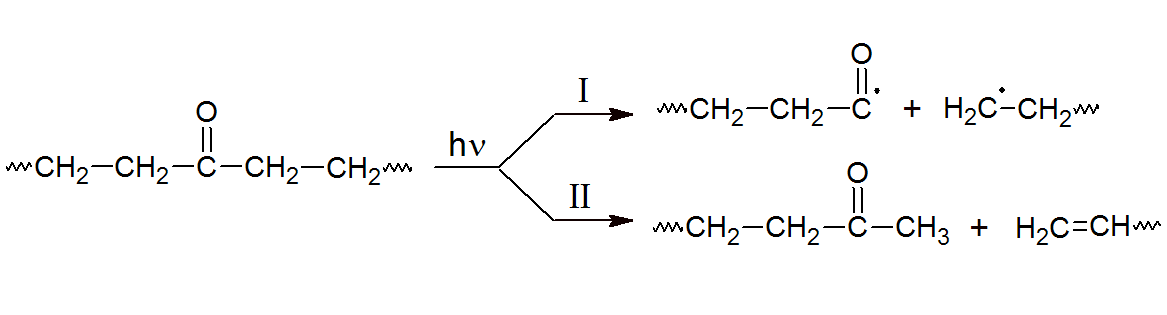
The Norish 1 reaction leads to chain scission and formation of radicals that might then initiate photo-oxidation. However, in the case of polyethylene, homolytic cleavage processes (Norish I) are only responsible for a small percentage of the chain breaks at room temperature.
References & Notes
- Chemical groups that are capable of absorbing UV light.
- F. H. Winslow, Pure & Appi. Chem., Vol. 49, pp. 495 - 502 (1977)